First interpretations on the fermentation process itself appear only in the era of alchemists-scientists, the seventeenth century. It is the historical moment in which the investigation of the world becomes more profound, though still mixing irrational aspects with burning science.
The understanding of this process began with the observation that a substance is formed by fermentation: alcohol (even if it was not called that at the time). The identification of this substance has its roots in distillation and alchemy.


The oldest stills date back to the 2nd century BC, found in Mesopotamia and in Egypt. In ancient times they were used only to produce essences for perfumed balms or for medical purposes. The distillation of alcohol, to produce hydroalcoholic solutions, probably began with the Arab alchemists Rhazes (Abū Bakr Muhammad ibn Zakariyyā al-Rāzī) and Avicenna (Ibn Sīnā) between the 10th and 11th centuries. This knowledge was collected and disseminated in the West especially by the Schola Medica Salernitana, from the tenth century onwards.
In the thirteenth century the German philosopher and bishop Albertus Magnus describes in his works the substance “aqua ardens” obtained from the distillation of wine, so light to float on oil. Aqua ardens, burning water, and spirit of wine are among the most used terms for centuries to indicate alcohol or a more or less concentrated hydroalcoholic solution. Spirit derives from the Latin “spiritus” which means breath, vital spirit and, by extension, exhalation. The word alcohol will come later. Alcoholic beverages were indicated in this period with the generic term of aqua vitae (water of life), whatever the starting material.
The first work that describes in detail how to produce aqua vitae (with a double fermentation system) is a part of the Vatican code Consilia (1276). It is by the Florentine doctor Taddeo Alderotti, one of the most famous of his time, also mentioned in Paradise by Dante (12:82-85). I
Initially distillation of aqua vitae was carried out only in monasteries but, within a century, became increasingly popular. It was widespread mainly thanks to the treaty of the Paduan doctor Giovanni Michele Savonarola (1385-1468), the “De conficienda Aqua Vitae“, which explained how to prepare it (he is the grandfather of the famous Dominican friar Girolamo Savonarola).
Then by empirical distillation practice, increasingly perfected, it was born the identification of the substance that characterizes the wine from the grape-juice. Based on this knowledge, in the seventeenth century, we find the first attempts to interpret fermentation.
The seventeenth century is an era that represents a important break in the history of humanity and science, thanks to the birth of the experimental method. This will allow to overcome the principle of authority over time and to abandon gradually the mystical and magical aspects of alchemy.
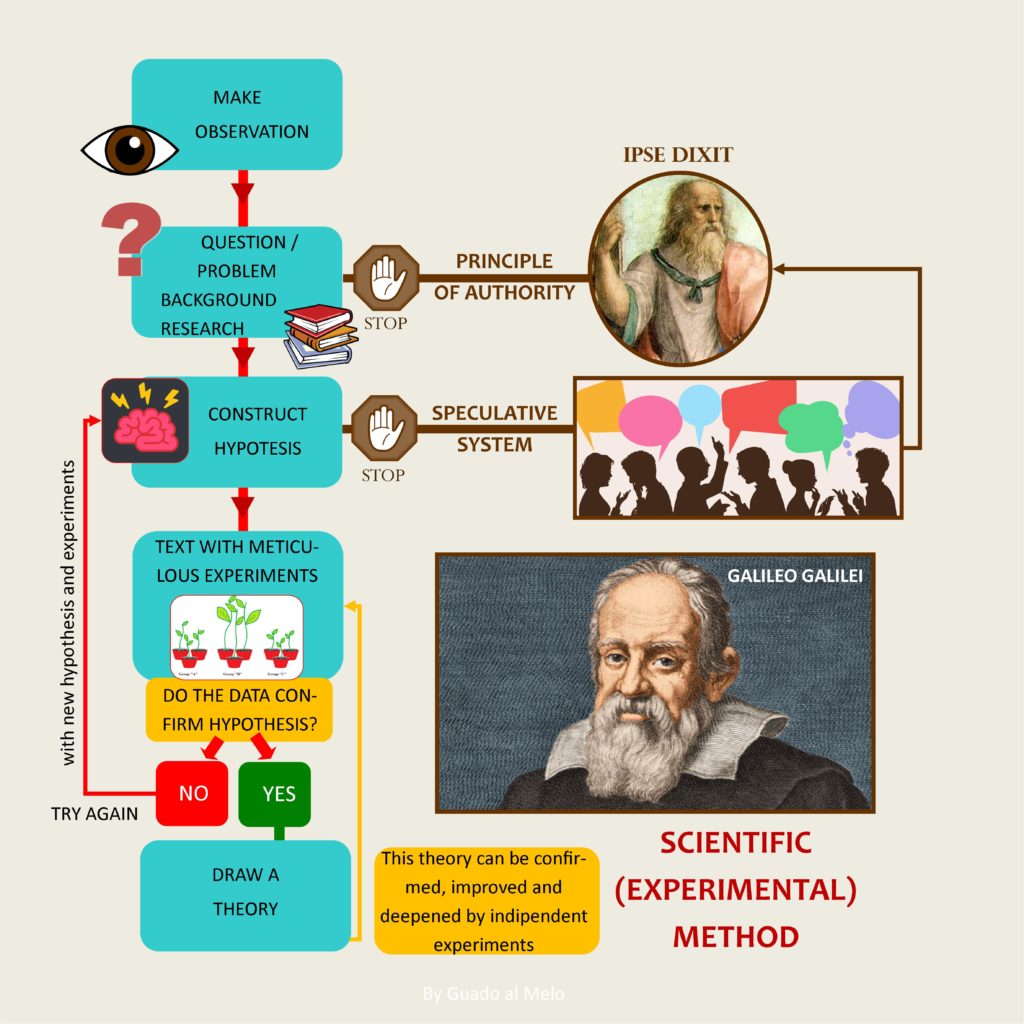
Naturally it was not a clean break. For a few more centuries science remained in a middle world, poised between rationality and magical thought, with different figures of cutting-edge scientists and many still linked to the past. Even those scientists, which we remember today for important discoveries, still maintained (to a greater or lesser extent) some magical or irrational aspects.

Returning to our topic, at the end of the sixteenth century the physician and chemist Angelo Sala (1576 – 1637) made the first studies on the formation of alcohol from fermenting musts.
Shortly afterwards the Flemish doctor and philosopher Johannes Baptiste van Helmont (1580-1644), disciple (and critic) of Paracelsus, admirer of the new experimental method, was among the first to hypothesize that fermentation was a chemical process, mediated by not well specified intermediaries. For van Helmont (and others of his time) reality consists of “minima naturalia“, very small elements, corpuscles at the base of all matter. Chemical reactions, which he call indistinctly “fermentations” or “effervescences”, are additions or subtractions of these particles. At the basis of the reactions there is a spiritual principle that operates through “ferments”, unidentified mediators.
In addition to this, van Helmont identified that the gas released during fermentation is the same that is obtained by burning coal, at the time called “gas silvestre”, sylvan gas (because it derives from the combustion of plant matter). Today we call it carbon dioxide.

Another exponent of this world in the balance between nascent science and alchemy is Nicolas Lémery (1645-1715), French chemist who, in the second half of the seventeenth century, considered five substances at the base of nature: Water, Spirit, Oil, Salt and Earth. His work “Course of Chemistry” (1687) was a great success and was long considered a reference text. we can find in it a detailed explanation of the process of alcoholic fermentation.
According to Lémey, vinification consists in the transformation of a part of the must (Oil) into alcohol (Spirit), thanks to an action of “rarefaction” by the Acid Salts.

For Oil he means the part that remains non-distillable, while the Spirit is the part that can be distilled and flammable. For Salts he means both those that remain encrusted on the walls of the barrels (tartaric acid) and those that precipitate on the bottom at the end of the vinification.

Lémery, with his language (sometimes obscure for us), begins to identify different elements present in the juice and in the wine, even if with wrong roles in fermentation. This explanation, however, will remain for a long time as a model. In fact we find it (much the same) in the “Dictionary of Chemistry” by Pierre Joseph Macquer of 1766.
In addition to this, however, he reasons that wine no longer has the sweet taste of juice. Furthermore, he says, fermentation takes place more easily and produces more spirit substance if grapes are ripe. The unripe and ripe grapes change in flavor: the first is more acidic and not sweet at all, the other is very sugary. Therefore he concludes that the ripening of grapes (and other fruit) consists in the accumulation of sugars, which become dominant. From here he deduces that the material that undergoes alcoholic fermentation is precisely sugar.
Macquer continues by explaining some traditional practices to increase the sugar in the grapes by concentrating them by drying. He also mentions the practice of adding sugar directly to the must. He also points out that as early as the beginning of the century the Dutch doctor Herman Boerhaave (1668-1738) indicated that sugar is an effective means of promoting fermentation. The practice of sugaring was then widespread among the French wine producers in the early decades of the nineteenth century, thanks above all to the chemist Jean-Antoine Chaptal (wine sugaring in French is called “chaptalisation“).
He writes, however, why it happen is still unknown and not easy to discover.
The first to describe clearly what happens during fermentation was Antoine-Laurent de Lavoiser (1743-1794) in his “Elementary chemistry treatise” of 1789. Lavoisier is one of those figures who changed history. He is recognized as the “father of modern chemistry” who, thanks to him, completely detached himself from alchemy.

Lavoisier begins to use the word alcohol and no more spirit of wine. In fact, he thought this term was not adequate because this substance could be formed not only from wine, but also from cider or sugar.

The word alcohol comes from the Arabic al-ko-ol. Originally it referred to the fine and black powder that women used to make up their eyes. From here, in Arabian alchemy, it was used to refer to any very fine powdered compound and so it also passed into the Western Middle Ages. It was then used to indicate the purest essence of a substance. It seems that the first to use the word alcohol in the modern sense was Paracelsus (Theopharst Bombast von Hohenheim, 1493-1541), the best known alchemist of his time. Lavoisier introduced it, however, into the common scientific use. In fact he is also remembered for his important reform of the nomenclature of chemical substances, at the base of the modern one. He wanted to go from the obscure and vague terms of alchemical origin to others, more concise and rational ones.
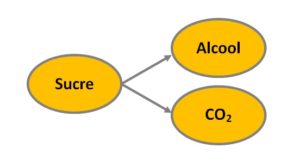
Returning to fermentation, Lavoiser, based on the knowledge accumulated up to his time, described it roughly as:
grape juice = carbonic acid (carbon dioxide) + alcohol
He experimentally reproduced the process with sugar dissolved in lukewarm water, to then estimate in a very precise way the quantities of fermented substances and the products obtained, also determining their composition. He was the first to write a chemical reaction as an equation. The measures were not exactly precise (given also the limits of the instruments of the time) but it was a real revolution for chemistry.
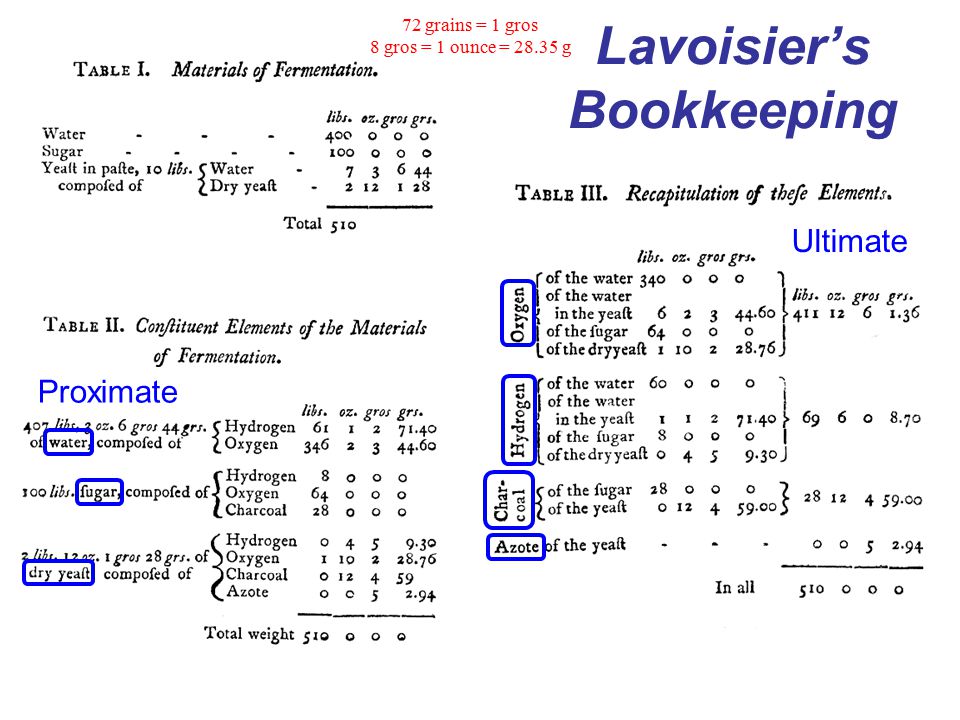
He described how the sugar split into two parts, and that the fermentation consisted in the oxygenation of one of them, with the formation of a combustible substance, alcohol. He showed that the transformation was not quantitatively exact because other secondary products are also formed.
The formula of the reaction was then defined quantitatively more precisely in 1810 by the French chemist Joseph Louis Gay-Lussac (1778-1850), thanks to the further improvement of the measuring instruments.
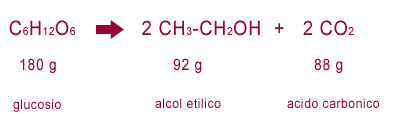
Then the chemists more and more refined the knowledge on the reaction, coming to define it with extreme precision. Today it is schematized as follows.
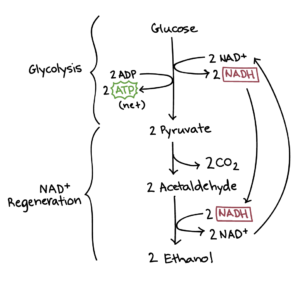

To get to this formula, however, still lacks a fundamental passage: at the time of Lavoisier there wasn’t still organic chemistry!
In fact, nobody has yet respond to that fundamental question that we have taken with us on this journey through the centuries: who does it and why?
This question also arose spontaneously by Vincenzo Dandolo, an Italian (contemporary) translator of the works of Lavoisier who, in addition to translating, also made numerous annotations to the text. Lavoisier in fact, describing his experiments, tells about using brewer’s yeast. Dandolo notes that the latter is formed by the sediment of the beer. He says that the fermentation is not well determined. He asks “why, how does brewer’s yeast work? … So the yeast becomes essential substance to make a complete fermentation, but though there are water, heat and sugar “.
So was yeast already known? No, this term is used to indicate something to facilitate fermentation empirically . Even from the ancient times, it was seen that by adding sediments of fermentation (or a bit of leavened bread dough in the case of bread making) the process started with greater ease. However, at the end of the eighteenth century, although having generally defined what happens, there was still no idea why.
Dandolo asks again: “What fermenting principle introduces this substance, or any other suitable to promote this first notable alteration …? Would this phenomenon refer to the nitrogen that the yeast  contains? If so, how does nitrogen work? ”
contains? If so, how does nitrogen work? ”
He says that the yeasts traditionally used are of two species:
- “highly putrescible bodies containing nitrogen” (he write how some facilitate fermentation by throwing pieces of meat into the vat; he also tells how the Chinese add excrement to stimulate the fermentation of a decoction of oats and barley .
- “Bodies that contain a lot of oxygen like acids, neutral salts, clay, metal oxides, etc.“
Beyond these aneducts and theories, for many at the time (as for the same Dandolo) “yeast” was some chemical compound. Only Pasteur, in the second half of the nineteenth century, put an end to this long diatribe, identifying it in a living organism.
However, Pasteur’s work was not born from nothing and it was a very difficult and very controversial goal.
(to be continued…)